Használati útmutató Rossmax NI60
Olvassa el alább 📖 a magyar nyelvű használati útmutatót Rossmax NI60 (4 oldal) a Gamestoel kategóriában. Ezt az útmutatót 29 ember találta hasznosnak és 4 felhasználó értékelte átlagosan 4.3 csillagra
Oldal 1/4
1
1.0Object
1.1
To test the performance of Rossmax NB60Q nebulizer
2.0Equipment List
2.1Rossmax NB60Q Nebulizer*2
2.2Rossmax Nebulizer kit*2
2.3Malvern Spraytec particle size analyer
2.4Marple 298 Cascade Impactor
2.5Chroma 61602 Programmable AC Source
2.6Shimadzu AUW120D microbalance
2.7A.P. Buck, Inc. Libra Plus LP-5 sampling pump
2.8SSI P51-6BarS-A-MD-20mA pressure meter
2.9Golden Mountain Enterprise Co. Ltd. F33L0096 flow meter
2.10 Humidity/Temperature Meter
2.11 Taiwan Biotech Co., Ltd 0.9% Saline solution
2.12 Atrovent Ipratropium Bromide
2.13
Atrovent Flixotide
2.14 AstraZeneca Terbutaline Sulphate
2.15 Ventoline (2.5mg) Salbutamol/Sulphate
2.16 Casio Timer
3.0Testing Items
3.1Aerosol Particle Size Distribution Testing(By Malvern Spraytec)
3.2Aerosol Particle Size Distribution Testing(By Marple 298 Cascade Impactor)
3.3Nebulization Rate Testing(Including drugs testing)
3.4Residual Volume Testing
3.5Reliability Test
4.0Testing Procedure
4.1Aerosol Particle Size Distribution Testing(By Malvern Spraytec)
4.1.1Each sample should be tested with 2.5ml 0.9% saline solution for 3 minutes.
4.1.2Add 2.5ml 0.9% saline solution into the nebulizer kit,
4.1.3Connect the nebulizer kit with NB60Q and put at the testing site, the nebulizer
kit’s outlet must be kept at 3.0 cm from the laser beam.
4.1.4Start recording Spraytec for more than 15 secs, then start NB60Q for testing.
4.1.5After 3.0 minutes have been reached, stop the NB60Q and then stop Spraytec.
4.1.6Checks Spraytec records
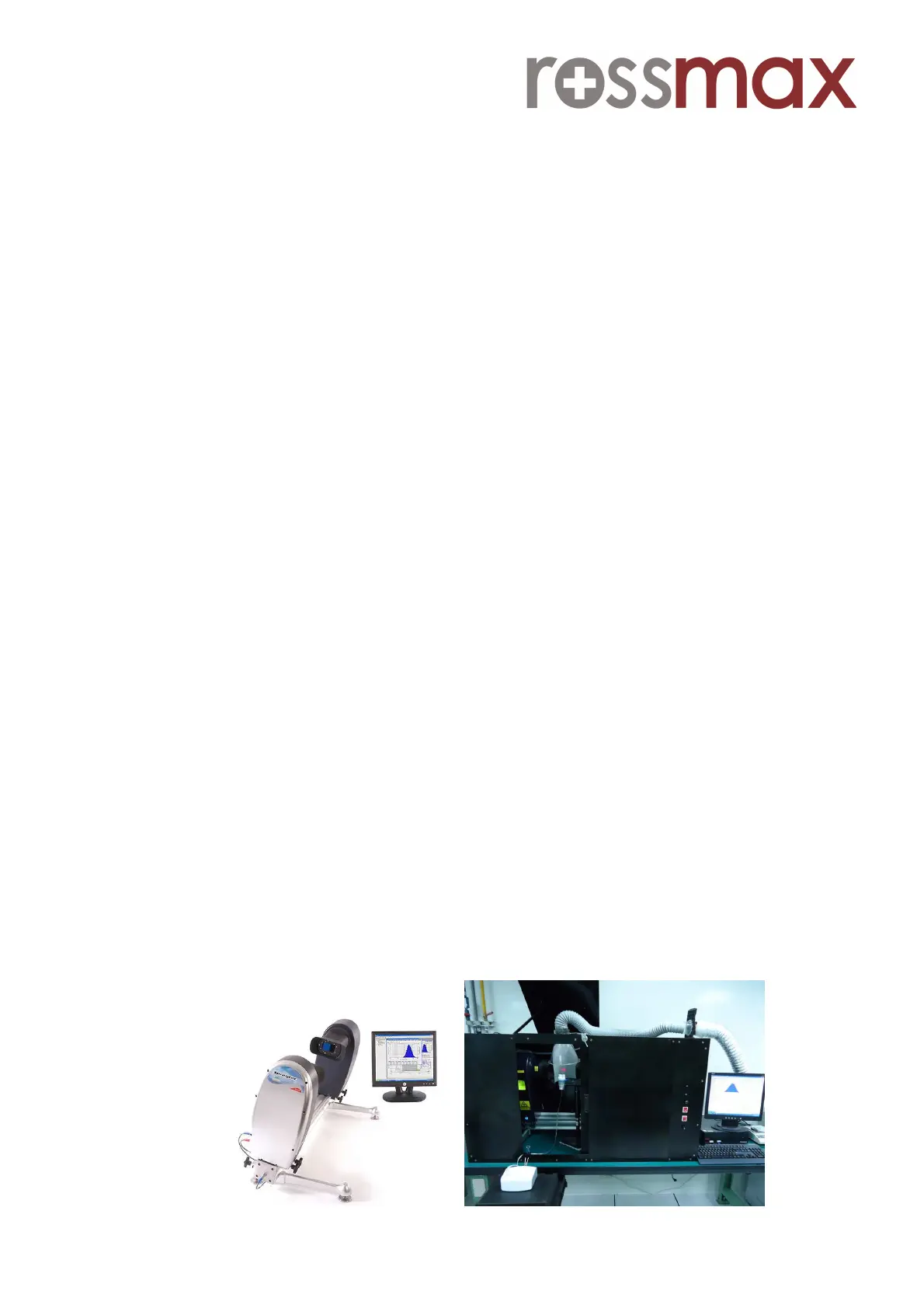
Fig 1. Malvern Spraytec and Testing site
Termékspecifikációk
| Márka: | Rossmax |
| Kategória: | Gamestoel |
| Modell: | NI60 |
Szüksége van segítségre?
Ha segítségre van szüksége Rossmax NI60, tegyen fel kérdést alább, és más felhasználók válaszolnak Önnek
Útmutatók Gamestoel Rossmax

30 Augusztus 2024

30 Augusztus 2024

30 Augusztus 2024

30 Augusztus 2024
Útmutatók Gamestoel
Legújabb útmutatók Gamestoel

8 Január 2025

28 December 2024

26 December 2024

26 December 2024

2 Augusztus 2024

2 Augusztus 2024

19 Július 2024

19 Július 2024

11 Július 2024